Thermal Energy
Heat Transfer
- Thermal energy (heat) transfer happens when there is a difference in temperature
- The energy moves from the higher temperature area to the lower temperature area
- Conduction
- Convection
- Radiation
Conduction
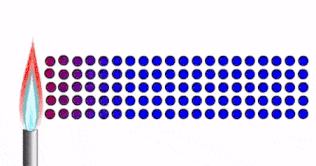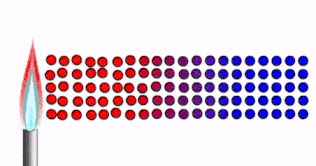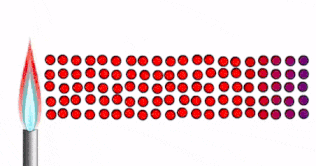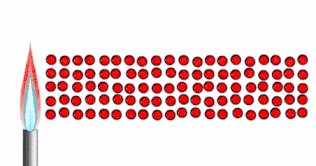
Conduction is how thermal energy travels through solids.
In a solid, the particles are tightly packed together in fixed positions.
- The particles are close together, vibrating in fixed positions
- As the solid is heated, thermal energy is transferred into kinetic energy in the particles.
- The particles vibrate faster
- The energy/vibrations are passed on from particle to particle
- Heat spreads through the solid
When you hold ice in your hand:
- Thermal energy moves from your hand to the ice
- Particle vibrations are passed from one particle to the next
- As your skin is losing thermal energy, it feels cold
Metals are good conductors of electricity. As well as vibrations being passed from particle to particle, as described above, metals also have ‘free’ electrons that can carry energy through the solid. This means that thermal energy conducts faster through metals than other materials.
Insulation
- Air is a poor conductor of heat as there are large distances between the particles
- This can be used to prevent heat loss from objects
Required Practical – Insulation
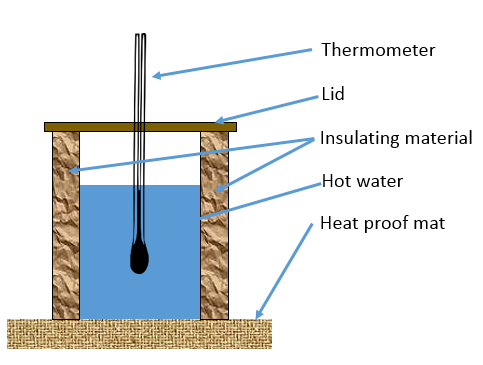
You need to be familiar with the practical method used to investigate the effectiveness of insulating materials.
- Hot water is placed in a container with a thermometer
- Insulating material is wrapped around the container
- The type of material, or different thicknesses of a particular material can be investigated
- The temperature is recorded over a period of time.
- The temperature change for different materials/thicknesses can be compared
Potential errors in this investigation include:
- Different starting temperatures of water can lead to different rates of heat loss each time
- Heat can be lost through the top/bottom of the container
- Random error can occur when reading the thermometer
Specific Heat Capacity
The temperature change of a substance depends on the following factors:
- The amount of thermal energy supplied
- The mass of the substance
- What the substance is
Different substances absorb thermal energy at different rates.
The specific heat capacity of a substance is the energy needed to raise the temperature of 1kg of the substance by 1oC
∆E = mc∆θ
∆E = energy transferred (J)
m = mass (kg)
c = specific heat capacity (J/kgoC)
∆θ = temperature change (oC)
(you will be given this equation on a data sheet in the exam)
Worked Example
0.15 kg of ice cream mixture was cooled from room temperature (20 °C) to 0°C. This required 6000 J of energy. What is the specific heat capacity of this ice cream?
Rearrange: c = ∆E ÷ (m∆θ)
c = 6000 ÷ (0.15x20)
c = 2000 J/kgoC
Storage Heater
A storage heater uses electricity at night (when it is cheaper) to heat bricks or concrete blocks inside the heater.
The bricks have a high specific heat capacity so they warm up slowly and then cool down slowly during the day, releasing thermal energy into the room. This is cheaper than using hot water in radiators but offers less control over the temperature of the room.
Required Practical – Specific Heat Capacity
You need to be familiar with the practical procedure used to find the specific heat capacity of a given material.
- Measure the mass of the aluminium block (or other material)
- Insert thermometer and record the temperature of the block (before heating starts)
- Use an immersion heater to heat the block for a certain amount of time
- The immersion heater should be connected to a joulemeter and the amount of energy supplied to the heater should be recorded
- Measure the temperature of the block after heating and calculate the temperature change
- Use the values recorded in your investigation to calculate the specific heat capacity
- Specific heat capacity = energy transferred ÷ (mass x temperature change)
The value you calculate for specific heat capacity is unlikely to be exactly the same as the published values for the same material.
This is due to errors in the investigation, including:
- The Joulemeter measures the electrical energy supplied to the heater. Not all of this energy will be transferred as thermal energy to the block – some will be lost to the atmosphere.
- Heat may be lost form the block to the surroundings during the experiment, this can be improved by insulating the block.
- Random error in reading the thermometer or balance
- What is the name for heat transfer in solids?
- conduction
- When a substance is heated, thermal energy is transferred to which type of energy in the particles?
- kinetic
- The temperature change of an object depends on the material, the thermal energy supplied and which other variable?
- mass
- What is the name for a material which does not conduct thermal energy very well?
- insulator