Rates of Reaction
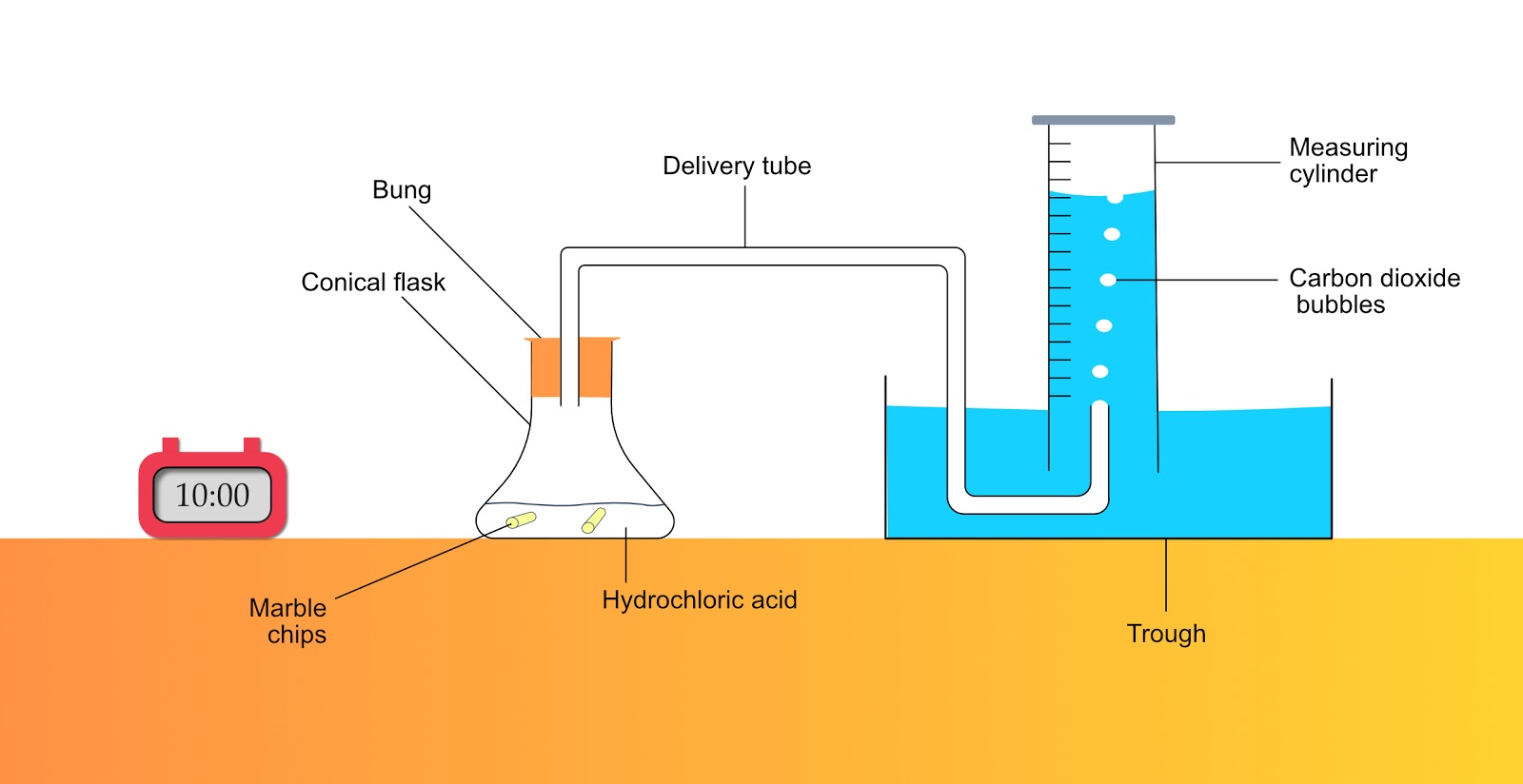
Gas Collection Method
The rate of a reaction can be changed by altering;
- Temperature
- Surface area or particle size
- Pressure
- Concentration
Reaction: Hydrochloric acid + Calcium carbonate → Calcium chloride + water + carbon dioxide
2HCl + CaCO3 → CaCl2+ H2O + ↑ CO2
During this reaction carbon dioxide is given off, this can be used to measure the time take for the reaction to occur and, therefore the rate of the reaction.
Safety: 1M HCl: Corrosive: handle with care, wear goggles.
Equipment:
- 0.5M, 1M and 2M HCl
- CaCO3 powder
- Marble chips
- Measuring cylinder
- Flask with bung and delivery tube
- Trough of water
- Timer
- Measuring cylinder 100 ml
Basic Method
Set up the equipment as shown.
The concentration of Acid
Use 2g of marble chips for this experiment. Add 25 ml of HCl starting with 0.5M acid time how long it takes to produce a set volume of CO2, say 30 ml. The amount of CO2, collected does not matter as long as the time to collect that amount is kept constant between experiments. Repeat the experiment using 1M and then 2M acid.
Fair test: Control the volume of acid, the mass of marble chips and the amount of CO2 collected between each experiment.
Accuracy: The accuracy of the results depends upon reading the scales on the measuring cylinder accurately.
Repeatability: Each acid concentration should be tested at least three times to find an average and check that the results are repeatable.
Surface Area
The same experiment can be conducted but only 1M HCl is used. The experiment is conducted using marble chips and then powdered CaCO3.
These experiments will give a time in seconds for the gas to be produced. To find the rate of the reaction, divide 1 by the time to get the rate.
Other Methods
Not all reactions produce a gas that can be collected in this case the time for the reaction to finish can be used to measure the speed of the reaction and then calculate the rate.
Example
Sodium thiosulfate and hydrochloric acid react together to form a precipitate. This is insoluble so the mixture gradually turns cloudy as the reaction proceeds. The container holding the mixture is placed on a piece of paper with a black X on it. The time taken for the cross to disappear when viewed through the reaction is recorded as the speed of the reaction. The concentration of the reactants can be altered to investigate how this affects the rate of the reaction.
With any of these experiments, it is important to only change one variable at a time, otherwise it would not be a fair test and the results would be invalid.