Alkali Metals
Physical Properties
The alkali metals from group 1 of the periodic table, (the first column not including hydrogen).
Properties
- Low density metals, lithium, sodium and potassium will float in water
- Very soft, can be cut with a knife for example
- Low melting points for metals
- Conduct electricity
Melting points decrease from lithium to francium
| Li | 180oC |
| Na | 98oC |
| K | 63oC |
| Rb | 39oC |
| Cs | 28oC |
| Fr | 27oC |
Chemical Properties
The main chemical properties of an atom are linked to the electron configuration of the atom. The protons and neutrons in the nucleus are held in place by strong nuclear forces and do not take part in normal chemical reactions. Bonds are formed by alkali metals by transferring the single electron in their outer orbit to a non-metal element, this then forms an ionic bond.
The electrons in the higher orbits are more energetic and because they are further from the nucleus they are held less firmly in place. This makes them more reactive and release more energy when they do react. Caesium is more reactive than lithium.
The reactivity of the alkali metals increases as you go down the group.
All alkali metals are highly reactive, many of them can only be stored under oil in a jar, if they are left exposed to the air they will react with the oxygen and start to burn. Only lithium can be stored in a normal sealed jar.
They all react with water to form alkali solutions of metal hydroxides.
Lithium + water → Lithium hydroxide + hydrogen
2Li + 2H2O → 2LiOH + ↑H2(g)
Potassium + water → Potassium hydroxide + hydrogen
2K + 2H2O → 2KOH + ↑H2(g)
Note that the formula for this reaction is the same for all alkali metals, francium however is so reactive that it is not found in a metal form.
The hydroxide compounds formed from these reactions are strong alkalis.
They all form base compound with oxygen.
Lithium + oxygen → Lithium oxide
4Li + O2 → 2Li2O
Sodium + oxygen → Sodium oxide
4Na + O2 → 2Na2O
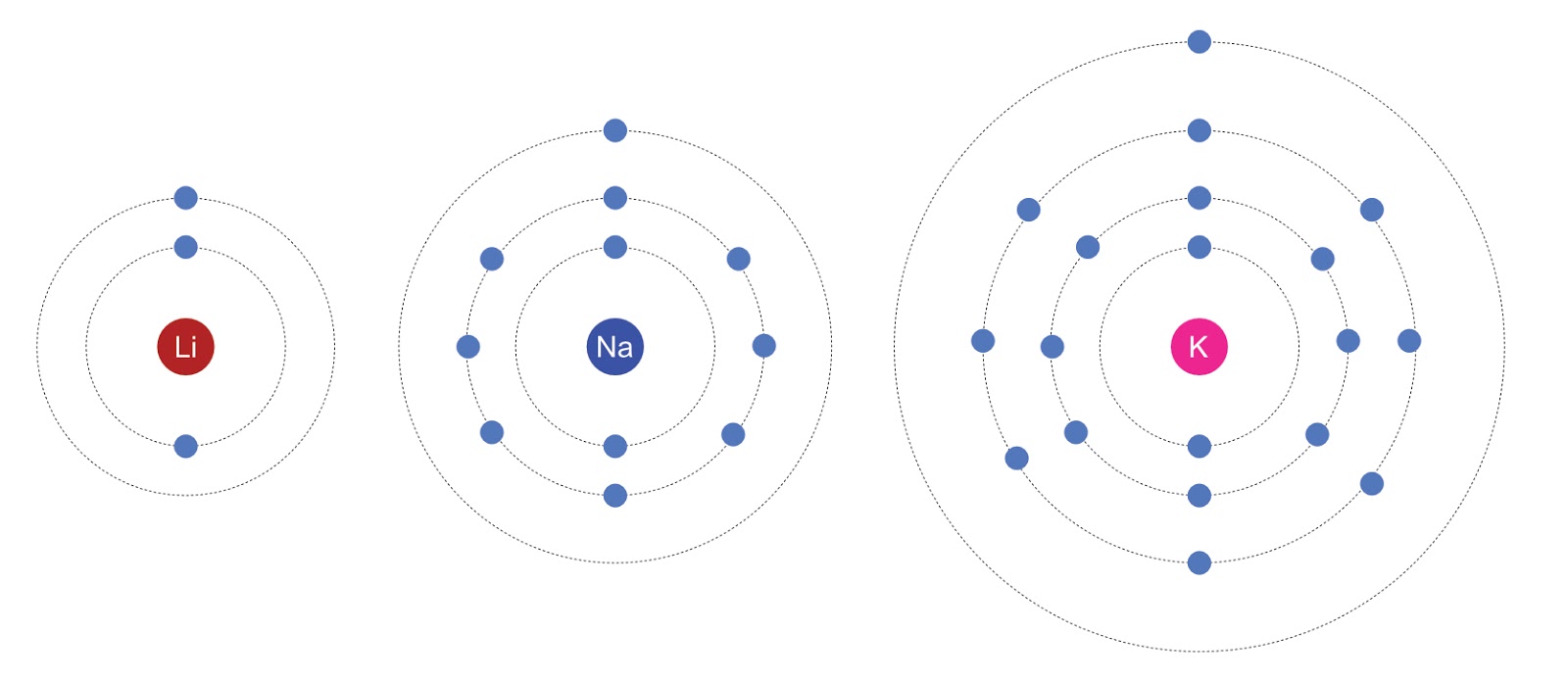
Electron Structures
All alkali metals have a single electron in their outer orbit, which is why they are all in group 1 of the periodic table. They increase in size and mass from top to bottom of the group.
It is the single electron in the outer orbit that determines their chemistry and makes them very reactive. As there is only one electron to loose in a reaction they form ions very easily compared to other metals.