The Structure of the Atom
Inside the Atom
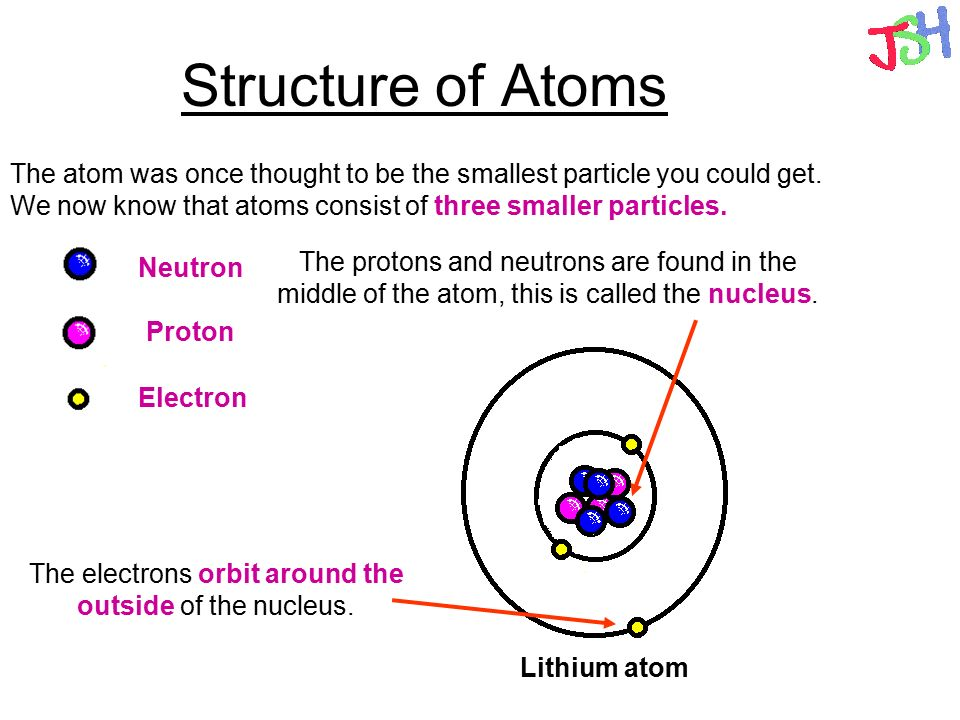
Atoms make up all matter in the universe, they are the building blocks of matter. Atoms, however, are not the smallest particles and even the building blocks have building blocks.
Inside the nucleus there are protons and neutrons. Protons have a positive charge (+1) and neutrons have a neutral charge (0). As a result the overall charge of the nucleus is positive.
Electrons have a negative charge (-1) and these are found around the nucleus of the atom in different energy shells.
The Size of an Atom
Atoms are incredibly small! In fact they are 1 x 10-10m or 0.0000000001m.
Atoms are mostly made up of empty space. The radius of a nucleus is around 10,000 times smaller than the radius of an atom.
Electronic Arrangments
The more energy electrons have, the further they are from the nucleus.
If an electron gains energy by absorbing electromagnetic radiation then they move further away from the nucleus.
They can also emit electromagnetic radiation (in the form of light waves) and as a result they move back closer to the nucleus.
- What particles are found inside the nucleus?
- Your answer should include: Protons / Neutrons
- What happens if an electron emits electromagnetic radiation?
- Your answer should include: Moves / Towards / Nucleus