History of Atomic Structure
Plum Pudding Model
You will need to know:
The basics of the plum pudding model of the atom.
So, here is some super exciting history!
Nuclear Model
You will need to know:
How the gold-leaf experiment worked and what conclusions were drawn from this.
How to draw, label and explain the set-up of the gold leaf experiment.
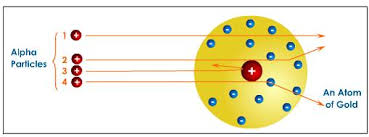
In 1909, Ernest Ruthorford disproved the plum pudding model by firing alpha particles through a very thin sheet of gold __(known as the __gold leaf experiment).
There are a few things you need to know before you can understand this experiment:
- Alpha particles are positively charged, made out of 2 protons and 2 neutrons
- Opposites charges attract (negative charges attract positive charges)
- Like charges repel (positive charges repel positive charges and negative charges repel negative charges)
When Rutherford shot the Alpha particles through a thin piece of gold, he saw 3 things happen (see the animation below):
- A few Alpha particles bounced back.
- Some alpha particles changed direction slightly.
- Some of the alpha particles passed straight through the sheet of gold.
Rutherford came up with a way to explain all of these observations:
- There is a tiny, solid mass in the middle of the atom - since some alpha particles hit this and bounced back.
- The solid mass in the middle of the atom is positively charged - since the positively charged alpha particles were repelled by the positive mass, making them deflect (change path).
- The majority of the atom is empty space - since most particles passed straight through without being affected.
- The negative electrons exist in a cloud around the outside of the nucleus - since the overall charge of the atom is neutral and the inside of the particle is positive.
Protons and Neutrons
You will need to know:
How Bohr’s nuclear model differed to previous models of the atom.
Scientists now know that electrons cannot just exists as a cloud around the nucleus, since it would cause the atom to collapse! Instead, it is now believed that electrons exist in energy shells.
The energy shells are filled up starting from the inner shell. Two electrons can fit in the first shell, with 8 electrons filling each shell after that.
Electronic Structure
You will need to know:
How electrons are now thought to occupy an atom.
How to write the electronic configuration for a given atom.
How to draw an atom.
Electrons occupy shells (energy levels) around the nucleus.
You will be drawing a lot of these electron shells in chemistry, and here are a few things to remember:
- The first shell can fit 2 electrons
- Every shell after that can fit 8 electrons
- The lowest energy levels are always filled first
- The electrons repel each other and like to be as far away from each other as possible
- After 4 electrons have been put into a shell, they start pairing up (apart from in th first shell).
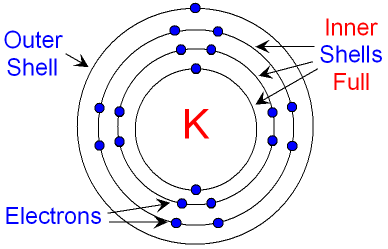
Example of a potassium (K) atom:
NOTE: Sometimes it is easier (and more useful) to write the atomic symbol where the nucleus should be instead of drawing all the neutrons and protons in the nucleus.
Electronic configuration:
(This sounds much scarier than it actually is!)
To save you from drawing out an atom every time they want to show how many electrons are in each shell, you can just write the electronic configuration. This is just a list of how __many electrons are in each she__ll!
For example, the configuration for the potassium atom above is: 2, 8, 8, 1.
How to draw an atom:
- Find an atom that you would like to draw on the periodic table. Let’s pick Sodium as our example.
- Write the symbol for that atom in the middle. Such as: Na
- Look at the atomic number on the periodic table, this is how many electrons there are! For sodium there are 11 electrons.
- Draw a ring around the symbol.
- Fill in the first ring with electrons, you can do this be drawing dots or crosses as electrons. Remember, there can only be a maximum of two in the first shell!
- If there are electrons left, draw a second circle.
- Keep filling shells and drawing extra shells until you’ve ran out of electrons!
- Your drawing should look like this!
- What is an alpha particle made up of?
- Your answer should include: 2 / Protons / Neutrons
- Is an atomic nucleus positively or negatively charged?
- Positively
- Write the electronic configuration for Magnesium.
- Your answer should include: 2 / 8
Explanation: 2, 8, 2