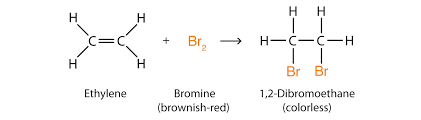Alkenes and Additional Polymerisation
Alkenes
When a hydrocarbon is unsaturated, it has some double bonds. These are called an alkenes.
Alkenes are more reactive than alkanes, since there is a ‘spare’ bond that could be made with another molecule.
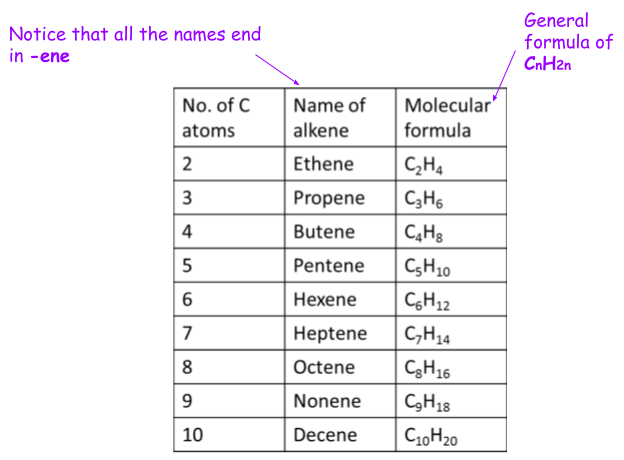
Naming alkenes
The same goes for __alkanes __(single bonds), except change the __-ene__ to an __-ane__!
Reacivity and Combustion of Alkenes
Combustion of alkenes
When alkenes are burnt in pure oxygen, they react with the oxygen to form carbon dioxide and water.
However, when they burn in air, they tend to incompletely combust:
You will need to know how to balance the equations for incomplete and complete combustion of alkenes and alkanes.
Addition reaction of alkenes
Addition reactions can happen with alkenes. This is when the alkene becomes saturated with other atoms to form only single bonds.
For example:
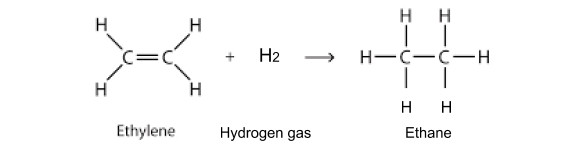
Hydrogenation of alkenes:
Thi is just a certain type of addition reaction involves adding hydrogen.
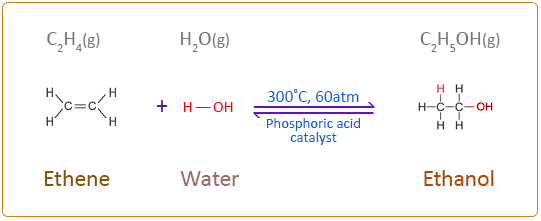
Forming Alcohols
An alcohol is very similar to a hydrocarbon in terms of its structure, except it has an alcohol group attached.
An alcohol group consists of an Oxygen and Hydrogen (O-H) bonded to one of the carbon atoms in the carbon chain. It’s always the oxygen that forms the bond with the carbon chain.
An alcohol can be made by reacting an alkene with water!
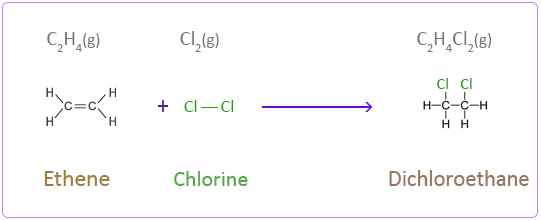
Addition of Halogens
As you may know from previous sections, a halogen is an atom that is found in group 7 on the periodic table! When an alkene is reacted with a halogen, it produces a haloalkane!
For example:
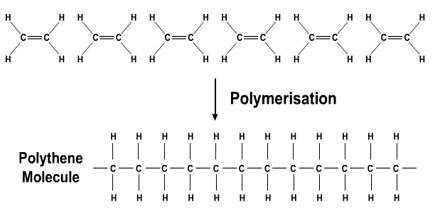
Additional Polymerisation
A polymer is a long chain of repeating units (monomers) Polymers are very useful since lots of plastics are polymers!
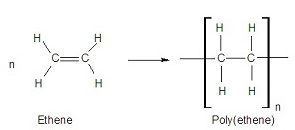
An additional polymer can be formed by putting a catalyst with lots of alkenes (all the same alkene, for example, ethene), at a high pressure. The alkenes all join together to form one long chain!
You can write the equation for this as:
The n is a number. If you had 100 ethene molecules reacting, there would be 10’s instead of n’s.
Naming polymers
It is very easy to name polymers, all you need to do is write ‘poly’, then the name of the monomer in brackets! For example: Poly(ethene), since this polymer is made from ethene monomers.