Metal Reactions
Reactivity Series
What you need to know:
What the reactivity series is and why it is useful
Know where carbon and hydrogen come in the reactivity series (even though they are not metals.
Why is it useful to label where carbon and hydrogen come on the reactivity series
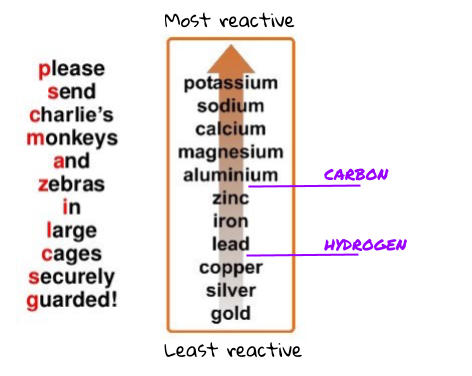
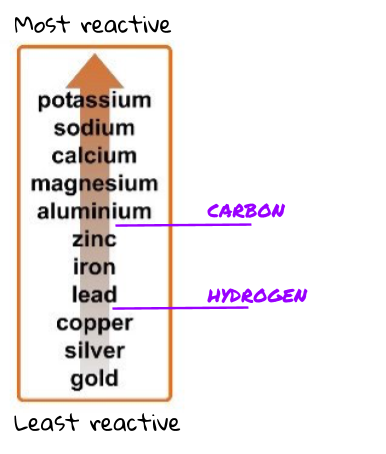
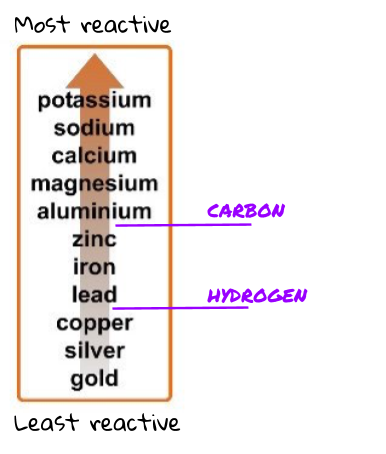
The reactivity series is just a list of metals. The metals in this list are ordered from most reactive to least reactive!
There are 3 main things to know about the picture of the reactivity series above.
- The rhyme will help you to remember the order, but in most questions you will be given the reactivity series.
- The top metal is the most reactive and the bottom metal is the least reactive.
- Carbon and hydrogen are not metals (so they are not actually in the reactivity series) but since they are two very common and useful elements, we usually mark on their place if they were to be in the reactivity series. For example, carbon is more reactive than zinc but less reactive than aluminium .
The order of the reactivity series can be worked out experimentally. You have probably done this in class. When you put each of the metals with either acid or water, you can easily see which ones have reacted most vigorously. Put these in order from most reactive to least reactive, and you have the reactivity series!
Reactivity of Metals with Acids
What you need to know:
How acids react with metals
How to test for hydrogen gas
When a metal reacts with acid, it produces a salt and hydrogen gas. The more reactive the metal, the more hydrogen gas is produced per second. Therefore the more reactive the metal, the more bubbles being produced!
Potassium, sodium, lithium and calcium react so vigorously with acid that there is an explosion! The less reactive metals (such as magnesium and aluminium) will just produce a lot of hydrogen gas.
How to test for hydrogen gas
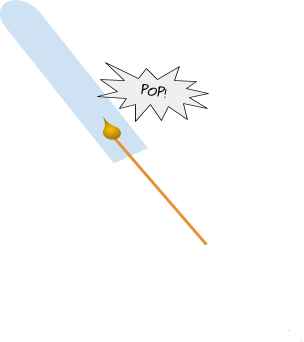
You can test for hydrogen gas using the squeaky pop test! To do the squeaky pop test, first you need to put acid with the metal into a test tube. You will see that the two start to react. You then need to catch the gas being released in another test tube, for a minute or so. Place a lit splint into the test tube. You should hear a loud squeaky pop sound if hydrogen is present!
The more hydrogen there is, the louder the pop sound!
Salts Formed
What you need to know:
How to name salts that are produced when acids and metals react.
The salt is very easy to name, if you have the name of the reactants. The metal will tell you the first half of the name, and the acid will tell you the last half!
For example:
Metals with Water
What you need to know:
How metals react with water and the equations to represent these.
Some very reactive metals, such as calcium and potassium can react with water to form a metal hydroxide and hydrogen.
Metals that are lower down on the reactivity series, such as zinc, iron, lead and copper won’t react with water. This is why it’s safe to have copper water pipes!
Example:
Displacement
What you need to know:
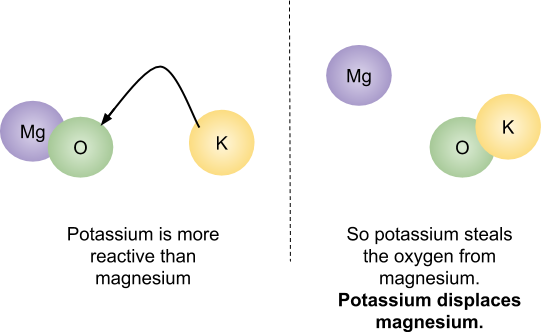
What a displacement reaction is and why one may occur.
A displacement reaction is when a more reactive meta metal replaces a less reactive metal in a compound. If the non-metal substance is already bonded to the most reactive of the two metals, there is no reaction. For example,