Group 7
Group 7Trends
What you need to know:
The properties of group 7 elements.
Group 7 elements are known as the halogens.
Properties of the halogens:
They are diatomic (travel in pairs, i.e Br2)
They are coloured vapours at room temperature:
- Fluorine is a poisonous yellow gas
- Chlorine is a poisonous green gas
- Bromine is a poisonous brown liquid or orange gas.
- Iodine is a poisonous grey solid or a purple gas.
Group 1Reactions with Metals and Non Metals
What you need to know:
How group 7 elements react with metals and nonmetals.
How melting and boiling points increase down group 7 and why.
How relative atomic mass increase down group 7 and why.
Reactivity decreases down the group. This is because group 7 elements react by gaining an electron. As you move down the group, the amount of electron shielding increases, meaning that the electron is less attracted to the nucleus. For this reason, fluorine is the most reactive halogen and astatine is the least reactive of the halogens.
Melting and boiling points:
Melting/boiling points increase down the group. This is because the atomic radii increase down the group, increasing the amount of intermolecular forces holding each molecule together.
Relative atomic mass:
Relative atomic mass increases down the group. This is because group 7 elements react by gaining an electron. As you move down the group, the amount of electron shielding increases, meaning that the electron is less attracted to the nucleus.
Reactions with non-metals:
Halogens form covalent bonds with other non-metal atoms when they react. This is a sharing of electrons.
Reactions with metals:
Halogens form ionic bonds with other metal __atoms __when they react. This is a giving/taking of electrons. The halogen atom takes an electron from metal atom.
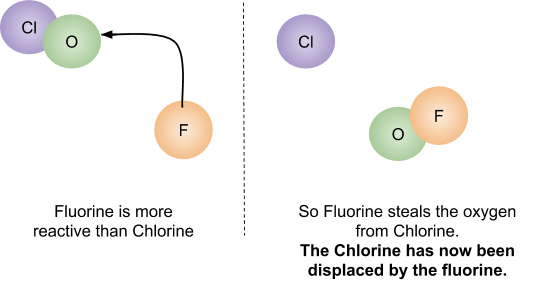
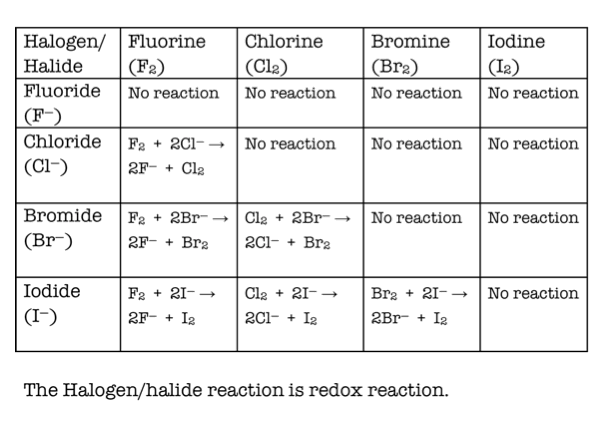
Displacement Reactions
What you need to know:
What a displacement reaction is.
Be able to work out if a displacement reaction will occur between a halogen compound and a different halogen molecule.
Be able to write the equation for any displacement reactions that may occur between halogen compounds and halogen molecules.
A more reactive halogen will replace another in a compound. For example, fluorine will displace chlorine in hydrogen chloride to produce hydrogen fluoride. If it is the more reactive molecule that is in the compound, no reaction will occur.
- Describe the look and nature of Fluorine at room temperature.
- Your answer should include: Diatomic / Poisonous / Yellow
- Describe the look and nature of Chlorine at room temperature.
- Your answer should include: Diatomic / Poisonous / Green
- Describe the look and nature of Bromine at room temperature.
- Your answer should include: Diatomic / Poisonous / Brown