Group 1
Group 1Trends
What you need to know:
- The trends in the properties of group 1 elements
- Why these trends exist.
- You need to know this for groups 0, 1 and 7 (0 and 7 are in different sections, but the reasons are pretty much the same).
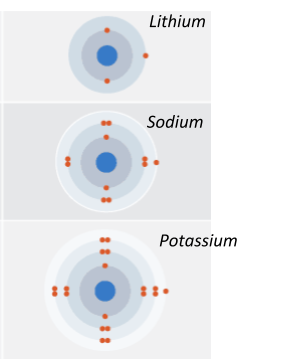
As you may (or may not) remember, all group 1 elements have 1 electron in their outer shell. This doesn’t mean that they all have the same amount of electrons, since they all have a different amount of shells (i.e they’re in a different period).
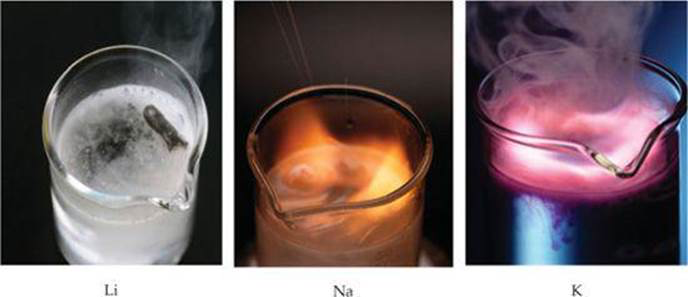
Here are the first three:
Trends
A trend is basically a pattern of behaviour.
So here are a few trends of group 1 (and the reasons why):
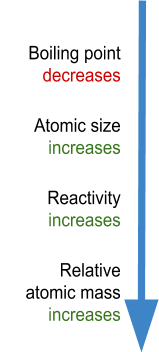
- Atomic size increases down group 1 - this is because the amount of electron shells is increasing! You can see this clearly in the picture above.
- Relative atomic mass increases down the group - this is because, not only are the amount of electrons increasing down the group, but (more importantly) the amount of protons and neutrons are increasing down the group!
- Reactivity increases down the group - This is because the group 1 metals react by losing an electron. As you go down the group, the outermost electron (negatively charged) gets further and further away from the positive nucleus. The nucleus is pulling all of the electrons towards itself, therefore if the electron is further away, it’s easier for the electron to leave!
- Melting and boiling points decrease down the group - So elements towards the bottom are more easily melted. Elements towards the top are more difficult to melt. You will learn why this happens in the bonding section.
Group 1Reactions with Water
What you need to know:
How group 1 elements react with water.
How to test if hydrogen gas is present
Group 1 elements react with water to form a metal hydroxide and hydrogen gas. Since group 1 elements are very reactive, they react with water very vigorously. The lower down in the group they are, the more reactive the metal (and hence the more vigorous the reaction).
Alkali Metal + Water -> Metal Hydroxide + Hydrogen
The metals react to form an alkaline solution and hydrogen. These metals are called alkaline metals since they react with water to form an alkaline solution!
You can use the squeaky pop test to prove that the gas being given off is actually hydrogen gas (see tests of common gases section). The really reactive alkali metals react with water with a bang! This is because hydrogen is very flammable and the reaction gives off energy (which ignites the hydrogen).
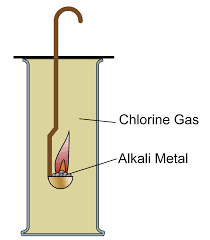
Group 1Reactions with Chlorine
What you need to know:
How group 1 elements react with chlorine gas.
How to react these two safely in the lab.
Group 1 metals react vigorously with chlorine to form white metal chloride salts.
Alkali Metal + Chlorine -> Metal Chloride
Reactivity increases down the group so the reactions get more vigorous.
Group 1Reactions with Oxygen
What you need to know:
How group 1 elements react with oxygen gas.
How to react these two safely in the lab.
Group 1Reactions with Non Metals
Group 1 metals react with oxygen to form metal oxides. The type of metal oxide that forms depends on which alkali metal is used and how much oxygen there is available for reaction.
__Alkali Metal + Oxygen Metal Oxide __
__Examples of oxides of group 1 elements: __
Li2O
Na2O
KO2
MgO
Notice that there are different ways in which oxygen can bind to group 1 elements (it may take two elements to bond to one oxygen or it may take one).