Using Resources
Ceramics and Composites
What you need to know:
State the different properties of ceramics
State the different properties of composites
Explain what a composite is and why they are useful.
Properties of ceramics
- Good insulators
- Brittle (shatter when broken) and stiff
- Non-metal
- High melting point
- There are clay and glass ceramics.
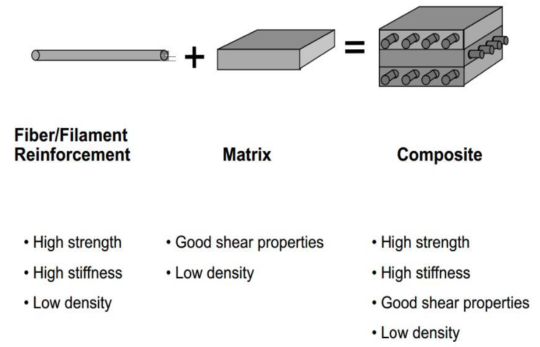
Properties of composites
Made of one material embedded in another
The properties depend on what the binder is made out of.
Polymers
What you need to know:
State the different properties of polymers
Explain what polymers are and why they are useful.
Explain the difference between thermosoftening and thermosetting polymers.
Properties of polymers
- Very large molecules with strong covalent bonds throughout.
- Insulators of heat and electricity
- Can be flexible
- Easily moulded
- Properties vary massively which means that a lot of different things can be made out of polymers, for example, clothes, plastic bags, wire cables etc…
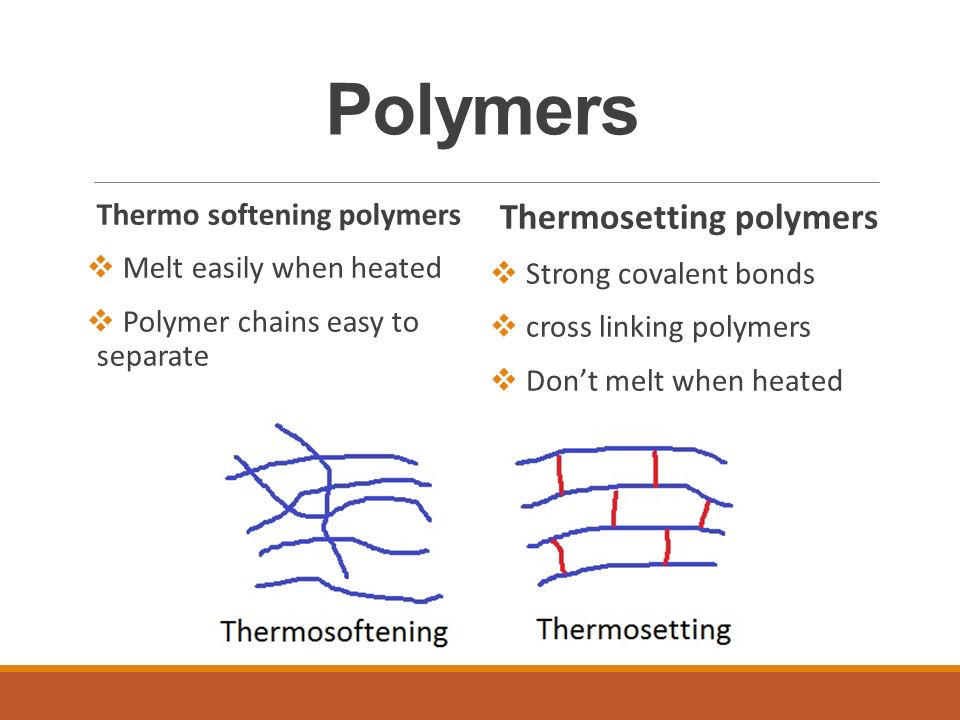
The type of polymer depends on what the monomers are made out of. The type pf bonds holding he polymer together can determine whether the polymer is thermosoftening or thermosetting.
__Thermosoftening __polymers are flexible since the forces holding the polymers together are weak.
__Thermosetting __polymers are strong, hard and rigid. They cannot be melted, instead they just char when burnt.
Metals and Alloys
What you need to know:
State the different properties of metals
Explain what an alloy is and why they are useful.
Properties of metals
- Malleable
- Good conductors of heat and electricity
- Ductile - can be drawn into wires
- Shiny
- Stiff
Alloys
A lot of the time, people want some properties of metals but not others. The most common example is that the strength and conductivity of metals is very useful when building bridges/machinery (etc) but the malleability is not wanted (you don’t want a bendy bridge!).
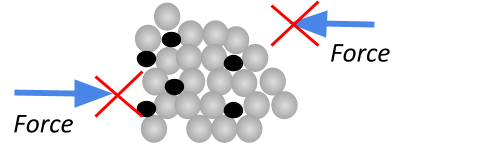
To solve this, alloys can be made. This is just where another type of atom is introduced into the metallic structure to jumble up the atoms. (If the atoms aren’t in a regular pattern anymore, they can’t easily slide over each other!). This makes the metals much stronger.
Carbon is a common example of the added substance