More on Acids
Strong and Weak Acids 1
What you need to know:
What is meant by the dissociation of acids.
What makes an acid weak or strong.
Dissociation of acids
When an acid is added to water (or an aqueous solution), the molecules within the acid let go of a H+, hydrogen ion.
For example:
Strong acids
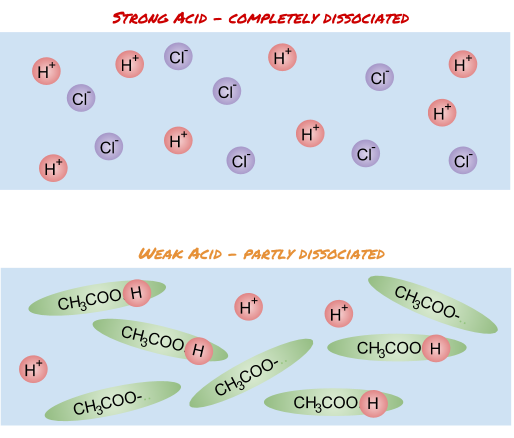
Strong acids are acids which completely ionise in aqueous solution. This means that all of the acid molecules lose a H+ ion completely.
Weak acids
Weak acids are acids which partly ionise in aqueous solution. This means that only some of the acid molecules lose a H+ ion.
Strong and Weak Acids 2
What you need to know:
How acid strength affects the reactivity of said acid.
How acid strength and pH are related
How acid strength affects reactivity
When acids react with other substances, it’s the H+ ion in the acid which is reacting with the molecules in the other substance. Therefore, the more H+ available, the more vigorously the acid reacts with other substances. For this reason, strong acids react more vigorously than weak acids.
Acid strength and pH
The pH scale is actually a measurement of the concentration of H+ ions within the acid. The higher the concentration of H+ ions, the lower the pH and the stronger the acid.
For every decrease of 1 on the pH scale, the H+ ion concentration increases by a factor of 10 (10x more than before).
You can work out how many more ____H+__ ions there are with the equation:__
Acid strength and concentration
Acid strength and concentration are two different things. The strength of the acid tells you what proportion of the molecules ionise in water. The concentration tells you how much of the solution is made up of the acid.
Acidic Reactions
What you need to know:
How acids react with different types of substances and the generic equations for each.
Reactions with metal oxides and metal hydroxides
__Difference between base and alkali: __
- Alkalis are substances that form OH- ions when they dissolve in water.
- A base is something that can neutralise acids.
- All alkalis are bases but not all bases are alkaline.
What do you call the salt?
The salt is very easy to name, if you have the name of the reactants. The metal (from the metal oxide or metal hydroxide) will tell you the first half of the name, and the acid will tell you the last half!
For example:
Metal carbonates reacting with acids
Metal carbonates are bases too. They reactant with acids to form a salt, water and carbon dioxide.
Just like before, the type of salt depends on the metal and the salt involved. The only difference is that now there is carbon dioxide released as well!
Making Soluble Salts
What you need to know:
How to make a soluble salt and how to purify the salt that is produced.
To make a soluble salt, you need to react an acid with a metal oxide/hydroxide/carbonate. You can work out which acid and base you will need by the name of the salt you want to form.
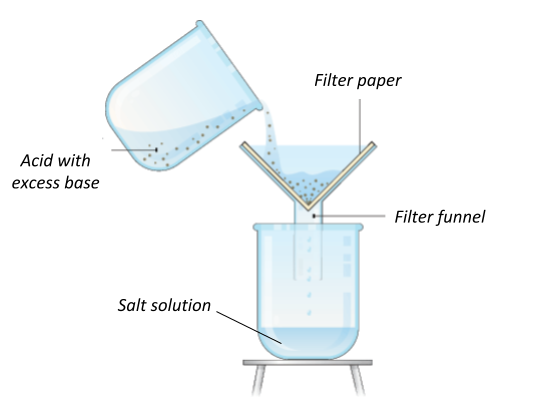
To form a soluble salt, you need to do the following steps:
- Put the acid in a beaker and gently warm the acid with the bunsen burner. Once the acid has been warming up for about a minute, turn off the bunsen burner.
- Place the insoluble base (metal oxide/carbonate) into the beaker and stir. You will see that the product is soluble.
- Keep adding the insoluble base until it is saturated. This happens when no more product can be formed and you will see that there will be some base left over which won’t react.
- Filter out the insoluble reactant using filter paper and a funnel. This will leave you with the salt solution.