Reversible Reactions
Reversible Reactions
What you need to know:
What is a reversible reaction?
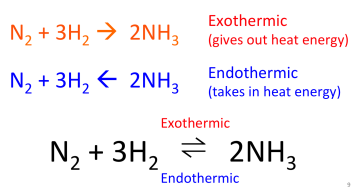
A reversible reaction is one that can also happen in reverse! So not only can the reactants react to form the products, but the products can also react to reform the reactants.
The double arrow means that the reaction is reversible.
Equilibrium
What you need to know:
What is a closed system?
What is equilibrium and how is it represented on a reaction rate graph.
Closed system:
A closed system just means that none of the atoms can get out and no more can get it. It’s basically a completely sealed container.
If a reversible reaction takes place in a closed system, it will reach an equilibrium.
Equilibrium:
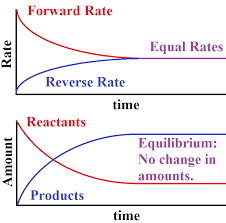
An equilibrium is when the reactants and products have formed a balance. The concentration of each stop changing.
- If two reactants are put together, they will start to react and products will form. The reactant concentration will fall and the products concentration will rise.
- As the product concentration starts to rise, the backwards reaction will start to happen. The more products there are, the faster the backwards reaction.
- The backwards reaction will decrease the amount of products and increase the amount of reactants.
- This continues to happen until they have reached a nice equilibrium. The forwards and backwards reactions have not stopped, but they are happening at a steady pace.
Le Chateliers Principle
What you need to know:
What is Le Chatelier’s principle
How changes in the environment of the system affect the equilibrium
Le Chatelier’s principle states that if a change is made to an equilibrium, the equilibrium will act to oppose the change.
Temperature change
- If you raise the temperature, the endothermic reaction will happen more.
- If you lower the temperature, the exothermic reaction will happen more.
Pressure
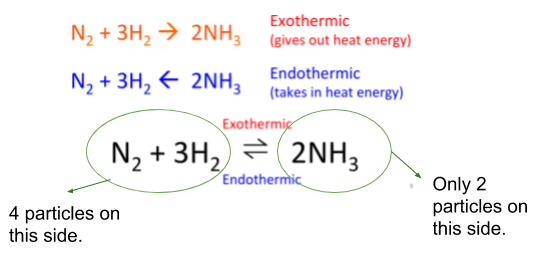
- If the pressure is raised, the reaction that produces less particles will increase its rate. This happens to try and lower the pressure to normal.
- If the pressure is lowered, the reaction that produces more particles will increase its rate. This happens to try and raise the pressure back to normal.
Concentration:
- If the concentration of the reactants is raised, the forward reaction will increase its rate in order to get the concentrations back to normal.
- If the concentration of the products is raised, the backwards reaction will increase its rate in order to get the concentrations back to normal.