Metallic Bonding
Metallic Bonding
What you need to know:
Know how metals bond with one another.
Structure of metals
The metal ions are arranged in a regular pattern. Metals are said to be giant structures since they usually contain lots of atoms.
Bonding in metals
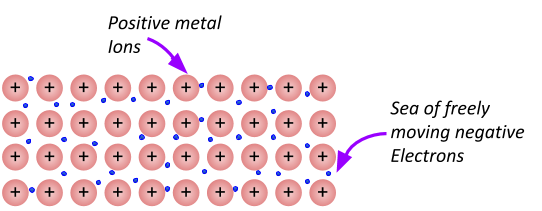
Metallic bonding forms between metals and metals. The extra electrons on the outer shell leave the atom, making the metal a positive ion. The electrons form a sea of electrons surrounding the positive metal ions. Since there are positive ions surrounded by a sea of negative electrons, it forms a bond around the ions (you can kind of think of the sea of electrons like the glue that holds the positive ions together).
Note
The amount of delocalised electrons depends on the amount of electrons there were in the outer shell of the metal atom. For example, magnesium has 2 electrons in its outer shell, so for every Magnesium atom that metallically bonds, the 2 electrons go off on their merry way to join the sea of delocalised electrons. This leaves the magnesium with a 2+ charge. So the bonding (force of attraction) in a lump of magnesium is twice as strong as the metallic bonding in lithium (which only has one electron on its outer shell).
A few things to remember:
- Metallic bonding happens between metals and metals
- The metal atoms lose their extra electrons on the outer shell
- Metal atoms become positive metal ions
- The extra electrons form a sea of electrons around the metal ions
Properties of Metals
What you need to know:
Understand how metallic bonding affects the properties of metals.
Understand what an alloy is and why they are useful.
Electrical conductivity
Metals are good conductors of electricity since they have a sea of delocalised electrons (they are free to move). The electrons can move to carry the charge (electricity) from one end of the substance to the other.
Thermal conductivity
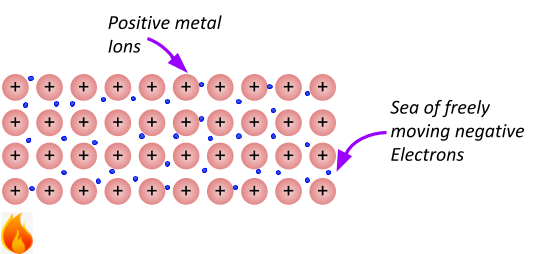
Metals are good conductors of heat since the ions are tightly packed and arranged in a regular pattern. Heat is just the vibration of molecules. When molecules heat up, they start to vibrate more and more. Heat travels through the substance by the particles bumping into and vibrating the ones next to them. This vibration passes through the substance, quickly heating up the whole of the substance.
High melting and boiling points
The force felt between the negative sea of delocalised electrons and the positive metal ions is called the electrostatic force. This force is very strong and requires a lot of heat to overcome. For this reason, metals usually have high melting and points! Making them solid at room temperature (normally).
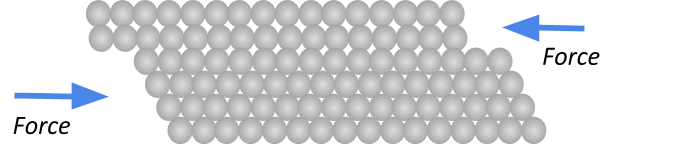
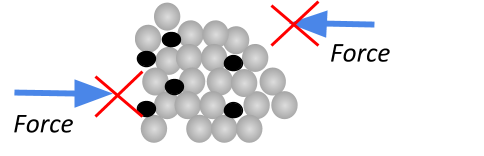
Malleability
If something is malleable, it just means that it can be easily moulded, bent, hammered or rolled into a different shape. Metals are malleable since the atoms are arranged in a regular pattern that can easily slide over each other!
Alloys
A lot of the time, people want some properties of metals but not others. The most common example is that the strength and conductivity of metals is very useful when building bridges/machinery (etc) but the malleability is not wanted (you don’t want a bendy bridge!).
To solve this, alloys can be made. This is just where another type of atom is introduced into the metallic structure to jumble up the atoms. (If the atoms aren’t in a regular pattern anymore, they can’t easily slide over each other!)
Carbon is a common example of the added substance: