History of the Periodic Table
Development of the Periodic Table
What you need to know:
How the periodic table used to be arranged
Why is arrangement was wrong.
How the discovery of isotopes proved that the first arrangement of the periodic table was wrong.
Neutrons, protons and electrons were not discovered until recently, so scientists could only classify elements by two different properties:
- Their chemical and physical appearance (how they look and how they react)
- Their relative atomic mass (how heavy they are)
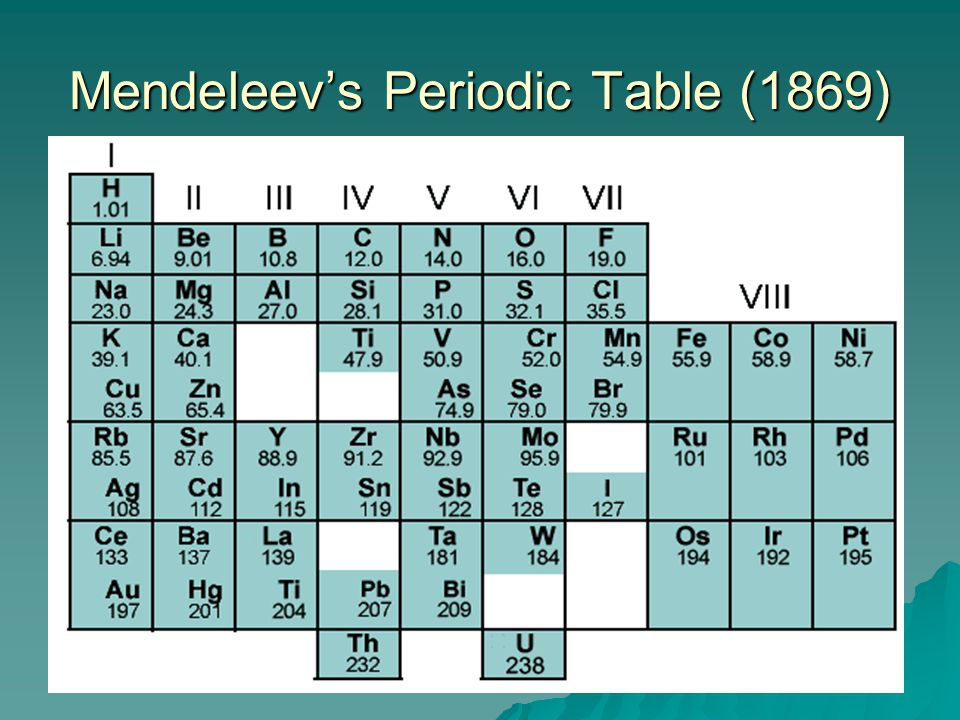
Before the 20th century, scientists arranged elements in order of their relative atomic mass into a table. When they did this, they realised that a pattern started to emerge and they called this the periodic table (since the patterns occurred periodically).
Since the early periodic table was arranged by atomic mass (instead of atomic number), a lot of the elements ended up in the wrong group, with elements that had different properties! Also, not all elements had been discovered, so there were gaps in the table.
Note: now we arrange them in terms of how many protons are in the nucleus.
Mendeleevs Periodic Table
What you need to know:
How the periodic table is now arranged
Why is arrangement works
In 1969, Dmitri Mendeleev re-organised the periodic table.
He kept the general pattern of relative atomic mass but made a few very important changes:
- He swapped some elements so that they were in groups that shared similar properties
- Gaps were left to allow for elements to be put into the right groups - this meant that the properties of undiscovered elements could be theorised!
The discovery of isotopes
In the early 20th century, isotopes were discovered (the same element but with a different mass). This proved mendeleev’s theory of arranging elements in order of mass AND their properties. This is because isotopes can have different masses but the same properties (so with the old periodic table, a heavy hydrogen might have been put in the wrong group!)
The Modern Periodic Table
What you need to know:
How the periodic table is now arranged
Why is arrangement works
What groups and periods represent.
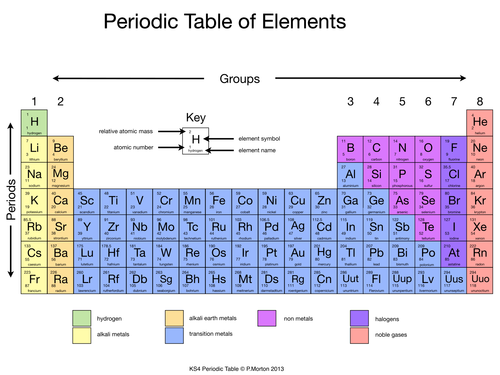
The periodic table is a table of all of the known atoms in existence, and it looks like this…
There is usually a key at the top of the table, which tells you what all the information means in the boxes:
As you can see, for this particular table (some look a LITTLE different), the atomic mass for each element is found at the top of each box, and the atomic number at the bottom. For all periodic tables, each box represents one atom.
Groups and Periods
You may notice that the table is labelled, with groups across the top and periods along the side. There are 8 groups and 7 periods, and each atom is assigned to a group and to a period. For example, Hydrogen (the green box with a ‘H’ inside) is in group 1, period 1. Neon (the pink box with ‘Ne’ inside) is in group 8, period 2.
Electronic Structure in the Periodic Table
What you need to know:
How the electron structure of elements changes throughout the periodic table.
The group number just tells you how many electrons the atom has on its outer shell, where as the period number tells you how many electron shells the atom has! This is what makes the periodic table SO USEFUL!
Here are some examples:
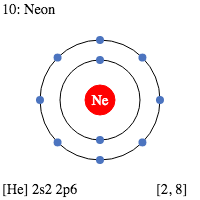
Here, you can see that Neon has 8 electrons on its outer shell (it’s in group 8) and 2 electron shells (period 2)! Hydrogen has 1 electron on its outer shell (group 1) and it has 1 electron shell (period 1).
Reactivity
What you need to know:
How reactivity of elements changes throughout the periodic table and why.
Reactivity just means: how likely it is that a particle is going to want to bond with (chemically join) another particle. All atoms aren’t happy unless they have a full outer shell of electrons; just like I’m not happy unless I’m full! If atoms don’t have a full outer shell of electrons then they’ll react with other atoms to try and get a full outer shell!
This means that:
- All atoms in group 8 have a full outer shell already and are stable. This means that they don’t react and they are happy as they are! This is sometimes called inert or noble. (Group 8 atoms are commonly referred to as ‘noble gases’.)
- Group 1 atoms usually react by losing one electron (this is because it’s much easier to lose 1 then gain 7).
- Group 7 atoms usually react by gaining one electron (this is because it’s much easier to gain 1 then lose 7).
You will learn more about this in the bonding section!
Metals and Non Metals
What you need to know:
What an ion is and how this changes the electron configuration of a particle.
Where metals and non-metals are found on the periodic table
How metals and non-metals differ in terms of properties.
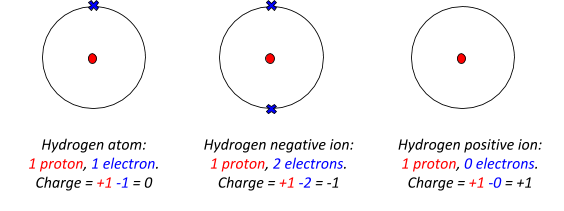
Ions
Before we dive into metals, you’ll need to know what an ion is. An ion is just a charged particle. This happens when atoms/particles lose/gain electrons. This means that they have more/less protons than they do electrons.
Here are two examples:
Metal atoms are atoms which form positive ions when they react.
Non-metal atoms are atoms which don’t form positive ions when they react. They either form negative ions by taking electrons from a nearby metal (in an ionic bond) or they share their electrons (in a covalent bond). If you’re a little unsure on this, don’t worry too much, it is explained in more detail in the bonding section.
If you’re lucky enough to get a colour coded periodic table, then you can easily see which atoms are metals and which are non-metals. If you have a black and white picture, then there will always be a line separating where the different colours would be. On the periodic table above, all of the atoms in yellow, orange and light blue are metals, the rest are non-metals.
Electronic structure of metals and non-metals
Notice that the metals are further to the left of the periodic table, whereas non-metals are further to the right. This is because metals have a low amount of electrons in their outer shell and non-metals have a lot! This makes a lot of sense, since metals are defined as atoms which form positive ions (lose electrons) when they react! Think of atoms as lazy creatures, they always do the easiest thing to be happy. It’s easier for an atom with 2 electrons in the outer shell to lose them (and form a positive atom) then to gain 6.
Physical properties of metals and non-metals
You probably already know what metals and nonmetals are like, but you’ll need to know what points will get you marks on an exam!
Here are the main points to mention for each:
__Metals: __
- They’re usually shiny
- They’re strong (hard to break) but malleable (can be easily bent/shaped)
- Great conductors of heat and electricity (both heat and electricity can pass through them easily)
- Usually have high melting and boiling points (you have to heat them up A LOT in order to melt them)
Nonmetals:
- They’re usually dull looking (not nice and shiny)
- They are usually more brittle than metals (snap easier)
- Usually have a__ lower melting and boiling point than metals__ (so they’re not always solid at room temperature).
- They don’t USUALLY conduct electricity (don’t let electricity go through them)
- They are normally__ less dense than metals__
If you don’t know what ‘dense’ means you can think of it as:
If you had 2 identical boxes, one is filled with feathers and the other with bowling balls. The one filled with bowling balls would be heavier! This is because bowling balls are more dense.
Groups and Periods
What you need to know:
How you can determine the chemical and physical properties of an element depending on where it is found in the periodic table.
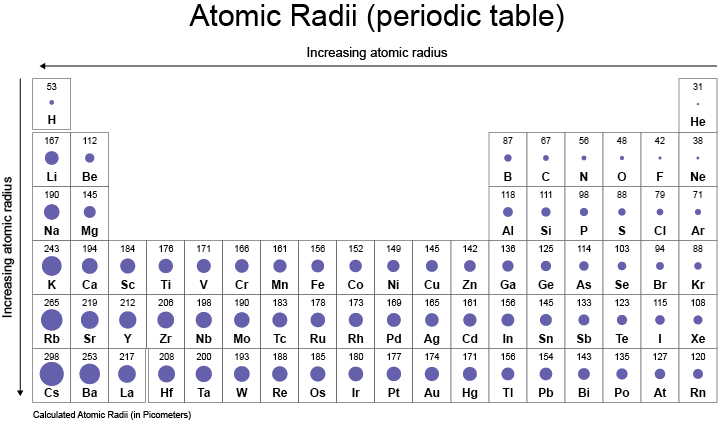
Atom size - The Periodic Table
You already know from previous lessons that the group number tells you how many electrons are in the outer shell, whereas a period tells you how many electron shells an atom has. These two things can affect:
- The size of the atom (see the picture above).
- How reactive the atom is.
- If the element of is monatomic, diatomic or otherwise. Basically, if they like to go around by themselves (like Helium), as a pair (like Hydrogen) or as a group (like potassium). You will only really talk about monatomic and diatomic elements.
You will learn WHY these three are affected in the next section. Before diving into that though, have a think and see if you can figure it out by yourself (I’d be very impressed if you do - young Einstein!)